Preclinical Testing of Inhaled Monoclonal Antibody for COVID-19 Shows Therapeutic Efficacy
Click HERE to see the full article on the Alabama NewsCenter website.
By Jeff Hansen
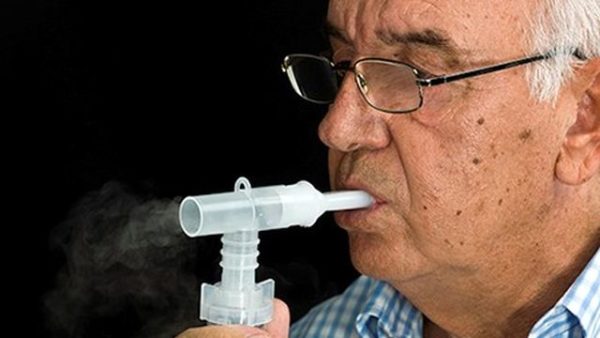
An inhaled treatment against the SARS-CoV-2 virus may lead to a future self-administered therapy for COVID-19, the pandemic that has killed more than 1 million people worldwide.
The treatment has successfully eradicated SARS-CoV-2 virus from infected animals at a 100-fold lower dose than other COVID-19 monoclonal antibodies reported to date. It was jointly discovered at UAB and the Texas Biomedical Research Institute, San Antonio, and has been licensed for development to Aridis Pharmaceuticals Inc., San Jose, California.
“Combining a highly potent monoclonal antibody with direct delivery to the lungs, which are the main target of the COVID-19 virus, achieved impressive efficacy in animal models,” said Dr. Hasan Jafri, chief medical officer at Aridis. “The therapeutic dose we observed corresponds to an estimated adult human equivalent efficacious dose of 1 to 3 milligrams. In contrast, other clinical stage COVID-19 monoclonal antibodies require up to 8,000 milligrams to achieve clinical benefits.”
The rest of the article is located at AlabamaNewsCenter.com.
Category: ALL POSTS, Partner News Stories